PROTECTION
Pre-fitted with a trocar-connected Minitulipe® infusion port with a breakable tamper-evident extension to protect the infusion site and reduce contamination risk3,4
Supplied in single-dose bags3
a ready-to-infuse formulation of gemcitabine in 10 SKUs3
steps in the preparation process, thereby removing opportunities for preparation error, contamination, and accidental exposure 1-6
error and contamination risk associated with gemcitabine compounding5
Select your dose
then Spike & Go™
Pre-fitted with a trocar-connected Minitulipe® infusion port with a breakable tamper-evident extension to protect the infusion site and reduce contamination risk3,4
Supplied in single-dose bags3
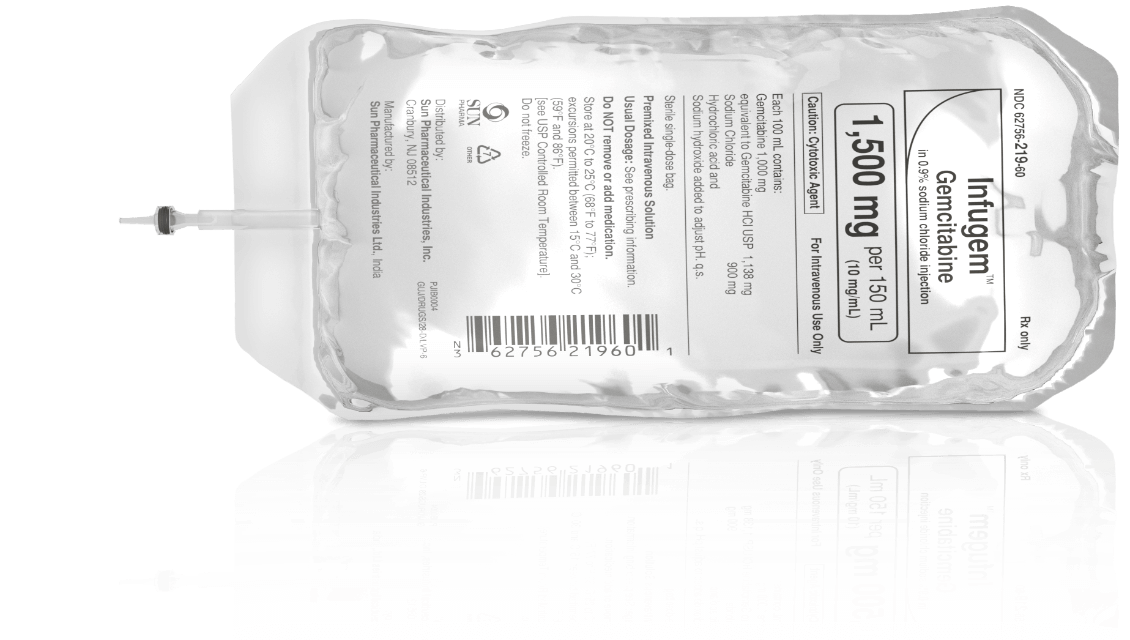

Packaged in aluminum pouches and hard-carton boxes to protect against accidental puncture and mechanical stress3





Final dosage formulation (10 mg/mL gemcitabine in 0.9% sodium chloride)—no drug manipulation required3






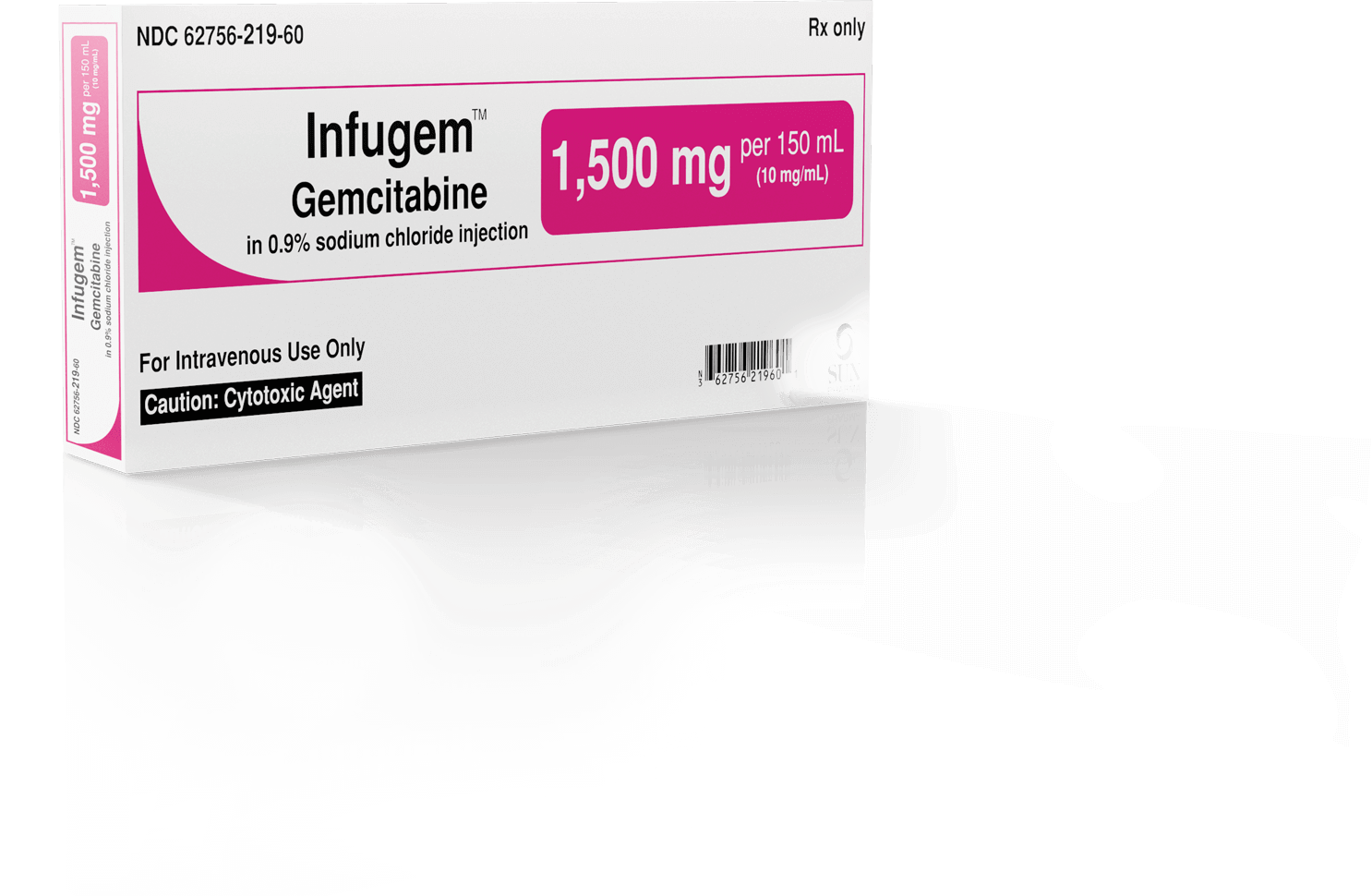
Color-coded and bar-coded packaging corresponds with each of the 10 different dosage strengths to facilitate dose identification3,4
Clear, easily understood, readable, unobscured labels approved by the FDA4
FDA=US Food and Drug Administration









CDC=Centers for Disease Control and Prevention.

INFUGEM is contraindicated in patients with a known hypersensitivity to gemcitabine. Reactions include anaphylaxis.
Schedule-Dependent Toxicity: In clinical trials evaluating the maximum tolerated dose of gemcitabine, prolongation of the infusion time beyond 60 minutes or more frequent than weekly dosing resulted in an increased incidence of clinically significant hypotension, severe flu-like symptoms, myelosuppression, and asthenia.
Myelosuppression: Myelosuppression manifested by neutropenia, thrombocytopenia, and anemia occurs with INFUGEM as a single agent. The risks are increased when INFUGEM is combined with other cytotoxic drugs. Monitor patients receiving INFUGEM prior to each dose with a complete blood count (CBC), including differential and platelet count, and modify the dosage as recommended.
Pulmonary Toxicity and Respiratory Failure: Permanently discontinue INFUGEM in patients who develop unexplained dyspnea, with or without bronchospasm, or have any evidence of pulmonary toxicity.
Hemolytic Uremic Syndrome: Hemolytic uremic syndrome (HUS), including fatalities from renal failure or the requirement for dialysis, can occur in patients treated with INFUGEM. Most fatal cases of renal failure were due to HUS. Serious cases of thrombotic microgangiopathy other than HUS have been reported with gemcitabine. Assess renal function prior to initiation of INFUGEM and periodically during treatment. Permanently discontinue INFUGEM in patients with HUS or severe renal impairment. Renal failure may not be reversible even with discontinuation of therapy.
Hepatic Toxicity: Drug-induced liver injury, including liver failure and death, has been reported in patients receiving gemcitabine alone or in combination with other potentially hepatotoxic drugs. Assess hepatic function prior to initiation of INFUGEM and periodically during treatment. Permanently discontinue INFUGEM in patients that develop severe liver injury.
Embryo-Fetal Toxicity: INFUGEM can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with INFUGEM and for 6 months after the final dose. Advise male patients with female partners of reproductive potential to use effective contraception during and for 3 months following the final dose of INFUGEM.
Exacerbation of Radiation Therapy Toxicity: INFUGEM is not recommended for use in combination with radiation therapy, either concurrently or ≤7 days apart. Life-threatening mucositis, especially esophagitis and pneumonitis occurred in a trial in which gemcitabine was administered at a dose of 1000 mg/m2 to patients with non-small cell lung cancer for up to 6 consecutive weeks concurrently with thoracic radiation.
Excessive toxicity has not been observed when gemcitabine is administered more than 7 days before or after radiation. Radiation recall has been reported in patients who receive gemcitabine after prior radiation.
Capillary Leak Syndrome: Capillary leak syndrome (CLS) with severe consequences has been reported in patients receiving gemcitabine as a single agent or in combination with other chemotherapeutic agents. Permanently discontinue INFUGEM if CLS develops during therapy.
Posterior Reversible Encephalopathy Syndrome (PRES): PRES has been reported in patients receiving gemcitabine as a single agent or in combination with other chemotherapeutic agents, and can present with headache, seizure, lethargy, hypertension, confusion, blindness, and other visual and neurologic disturbances. Confirm the diagnosis of PRES with magnetic resonance imaging (MRI) and permanently discontinue INFUGEM if PRES develops during therapy.
The most common adverse reactions for the single agent (≥20%) are nausea/vomiting, anemia, hepatic transaminitis, neutropenia, increased alkaline phosphatase, proteinuria, fever, hematuria, rash, thrombocytopenia, dyspnea, and peripheral edema.
Due to the potential for serious adverse reactions in nursing infants from INFUGEM, woman should not breastfeed during treatment with INFUGEM and for at least one week after the last dose.
The safety and effectiveness of INFUGEM have not been established in pediatric patients.
Please see Full Prescribing Information for INFUGEMTM here.
To report SUSPECTED ADVERSE REACTIONS, contact Sun Pharmaceutical Industries, Inc. at 1-800-818-4555, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
INFUGEM (gemcitabine hydrochloride in 0.9% sodium chloride injection) is a nucleoside metabolic inhibitor indicated for:
Ovarian Cancer: in combination with carboplatin, for the treatment of advanced ovarian cancer that has relapsed at least 6 months after completion of platinum-based therapy.
Breast Cancer: in combination with paclitaxel, for first-line treatment of metastatic breast cancer after failure of prior anthracycline-containing adjuvant chemotherapy, unless anthracyclines were clinically contraindicated.
Non-Small Cell Lung Cancer: in combination with cisplatin for the first-line treatment of patients with inoperable, locally advanced (Stage IIIA or IIIB), or metastatic (Stage IV)
non-small cell lung cancer.
Pancreatic Cancer: as first-line treatment for patients with locally advanced (nonresectable Stage II or Stage III) or metastatic (Stage IV) adenocarcinoma of the pancreas. INFUGEM is indicated for patients previously treated with fluorouracil.
1. Gemzar [prescribing information]. Indianapolis, IN: Eli Lilly and Company; 2018. 2. Gemcitabine [prescribing information]. Durham, NC: Accord Healthcare, Inc; 2018. 3. INFUGEM [prescribing information]. Cranbury, NJ: Sun Pharmaceutical Industries, Inc; 2020. 4. INFUGEM (gemcitabine in 0.9% sodium chloride injection) instructions for use. Cranbury, NJ: Sun Pharmaceutical Industries, Inc; 2018. 5. Urbine TF, Schneider PJ. Estimated cost savings from reducing errors in the preparation of sterile doses of medications. Hosp Pharm. 2014;49(8):731-739. 6. USP General Chapter <800> Hazardous Drugs – Handling in Healthcare Settings. http://www.usp.org/compounding/general-chapter-hazardous-drugs-handling-healthcare. Accessed January 6, 2020. 7. Centers for Disease Control and Prevention. Guideline for disinfection and sterilization in healthcare facilities (2008). CDC website. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/index.html. Updated May 2019. Accessed January 7, 2020. 8. Food and Drug Administration. Compounding and the FDA: questions and answers. FDA website. https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/PharmacyCompounding/ucm331 9. Centers for Disease Control and Prevention. Workplace Solutions: Personal protective equipment for health care workers who work with hazardous drugs. DHHS (NIOSH) publication 2009-106; October 2008. 10. Centers for Disease Control and Prevention. NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings. DHHS (NIOSH) publication 2004-165; September 2004. 11. Occupational Safety and Health Administration. Controlling occupational exposure to hazardous drugs. OSHA website. https://www.osha.gov/SLTC/hazardousdrugs/controlling_occex_hazardousdrugs.html. Accessed January 6, 2020. 12. The Joint Commission. Revisions to the medication management standards regarding sample medications. The Joint Commission website. https://www.jointcommission.org/assets/1/6/SampleMedications_HAP.pdf. Issued December 18, 2013. Accessed January 7, 2020. 13. Institute for Safe Medication Practices (ISMP). ISMP guidelines for safe preparation of compounded sterile preparations; 2016. ISMP website. https://www.ismp.org/tools/guidelines/IVSummit/IVCGuidelines.pdf. Accessed September 21, 2018. 14. American Society of Health-System Pharmacists. ASHP guidelines on preventing medication errors in hospitals. Am J Health Syst Pharm. 2018;75(19):1493-1517.
INFUGEM™ (gemcitabine in 0.9% sodium chloride injection) is available with a unique J-code for billable unit 100 mg
You are leaving the INFUGEM™
product website.
If you wish to continue,
please click OK.